
In simulations, arbitrary numbers of compartments with heterogeneous initial compositions segregate into a few compositionally distinct subsets that we term organelles.
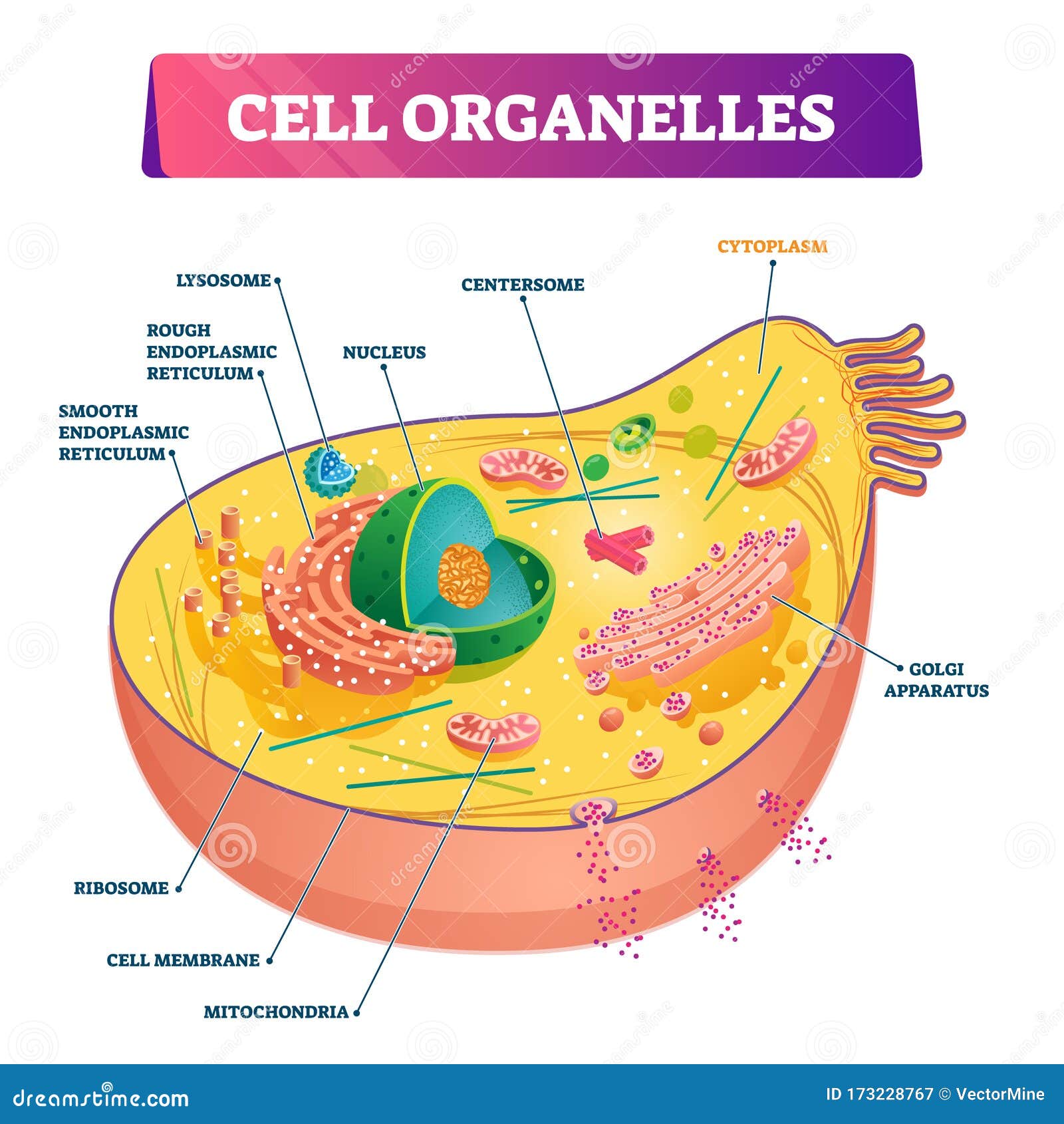
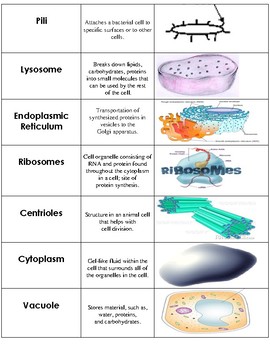
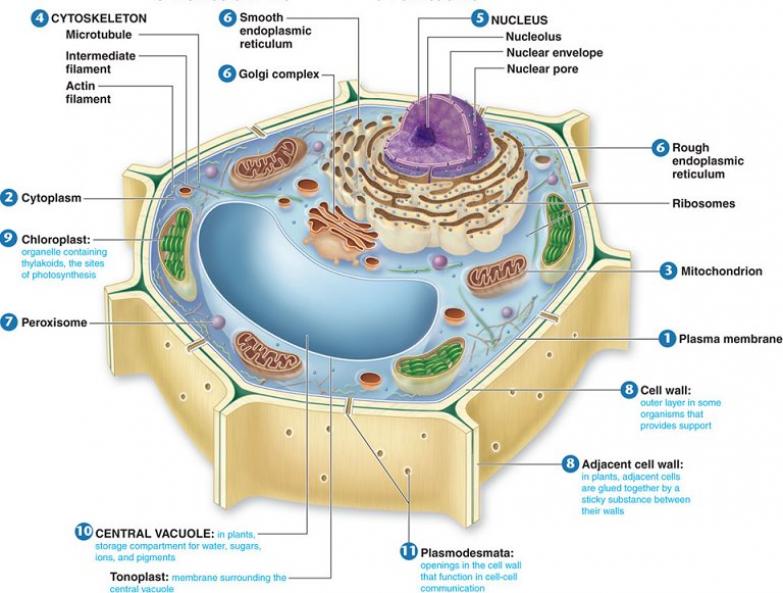
Our model incorporates membrane-bound compartments, coat proteins and adaptors that drive vesicles to bud and segregate cargo from source compartments, and SNARE proteins and associated factors that cause vesicles to fuse into specific destination compartments. Here we investigate the mechanistic link between gene duplication and the emergence of new nonendosymbiotic organelles, using a minimal biophysical model of traffic. This endomembrane system arose and diversified during a period characterized by massive expansions of gene families involved in trafficking after the acquisition of a mitochondrial endosymbiont by a prokaryotic host cell >1.8 billion years ago. Extant eukaryotic cells have a dynamic traffic network that consists of diverse membrane-bound organelles exchanging matter via vesicles.


 0 kommentar(er)
0 kommentar(er)
